Medical facilities face unique cleaning challenges that directly impact patient safety and regulatory compliance. Healthcare-associated infections affect 1 in 31 hospital patients according to CDC data.
We at Bumble Bee Cleaning Services understand the specialized protocols required for healthcare environments. This guide covers the standards, best practices, and selection criteria you need for effective medical facility cleaning.
Medical Facility Cleaning Requirements and Standards
Medical facilities must comply with three primary regulatory frameworks that define cleaning requirements. OSHA’s Bloodborne Pathogens Standard protects healthcare workers exposed to infectious blood, issued in 1991, and includes regulations for employers. Facilities must clean these areas immediately with EPA-registered disinfectants while staff wear proper personal protective equipment. The standard covers all areas where exposure might occur, including patient rooms, laboratories, and emergency departments.
CDC Environmental Cleaning Guidelines
The CDC Environmental Cleaning Guidelines provide current best practices for environmental cleaning procedures in patient care areas, as well as cleaning for specific situations. High-touch surfaces like bedrails, doorknobs, and medical equipment receive cleaning multiple times daily, while terminal cleaning after patient discharge requires comprehensive surface disinfection.
The CDC mandates that staff clean from clean to dirty areas and high to low surfaces to prevent cross-contamination. Blood or body fluid spills trigger immediate response protocols with approved disinfectants that have contact times from 30 seconds to 10 minutes (depending on the specific pathogen targeted).
Joint Commission Accreditation Requirements
The Joint Commission evaluates infection prevention programs during surveys and focuses on environmental cleaning documentation and staff competency. Facilities must maintain written cleaning schedules, assign specific responsibilities, and document task completion. Performance monitoring through visual assessments and microbial testing provides measurable compliance data.
The commission requires facilities to demonstrate staff training records, cleaning product safety data sheets, and evidence of regular protocol updates based on current infection control research. These comprehensive documentation requirements form the foundation for effective cleaning protocols that protect both patients and healthcare workers.
Best Practices for Medical Facility Cleaning
Chemical Selection and Contact Times
EPA-registered disinfectants must target specific pathogens present in healthcare environments. Quaternary ammonium compounds eliminate most bacteria and enveloped viruses with 30-second contact times, while bleach solutions destroy Clostridioides difficile spores after 1-minute contact periods. The EPA maintains List N with over 500 approved products for healthcare applications.
Hydrogen peroxide-based cleaners provide broad-spectrum effectiveness against resistant organisms like MRSA and VRE. These solutions require contact times from 30 seconds to 5 minutes (depending on concentration levels). Healthcare facilities must verify that staff apply products correctly and allow adequate dwell time for pathogen elimination.
High-Touch Surface Protocols
Door handles, bed rails, call buttons, and medical equipment need disinfection every 2-4 hours during patient occupancy according to CDC recommendations. Terminal cleaning after patient discharge involves comprehensive surface disinfection throughout the entire room.

Staff must replace cleaning cloths between each surface to prevent cross-contamination. Studies show that ATP monitoring detects residual organic matter on surfaces, providing immediate feedback on cleaning effectiveness through RLU level measurements that classify cleanliness as very clean, clean, equivocal, or fail.
Medical Waste Management
Red bag waste containing blood-soaked materials requires segregation at the point of generation and storage at temperatures below 50°F until disposal. Sharps containers need replacement when 75% full to prevent overfilling and potential injuries.
Washington State requires medical facilities to contract with licensed medical waste haulers who provide manifest tracking from collection to final treatment. Pharmaceutical waste demands separate collection streams with DEA-registered reverse distributors handling controlled substances through witnessed destruction processes.
Professional cleaning services with healthcare experience understand these complex waste streams and regulatory requirements. This expertise becomes essential when selecting partners who can maintain compliance while protecting your facility’s reputation and patient safety.
Choosing Professional Medical Cleaning Services
Certification Requirements and Training Standards
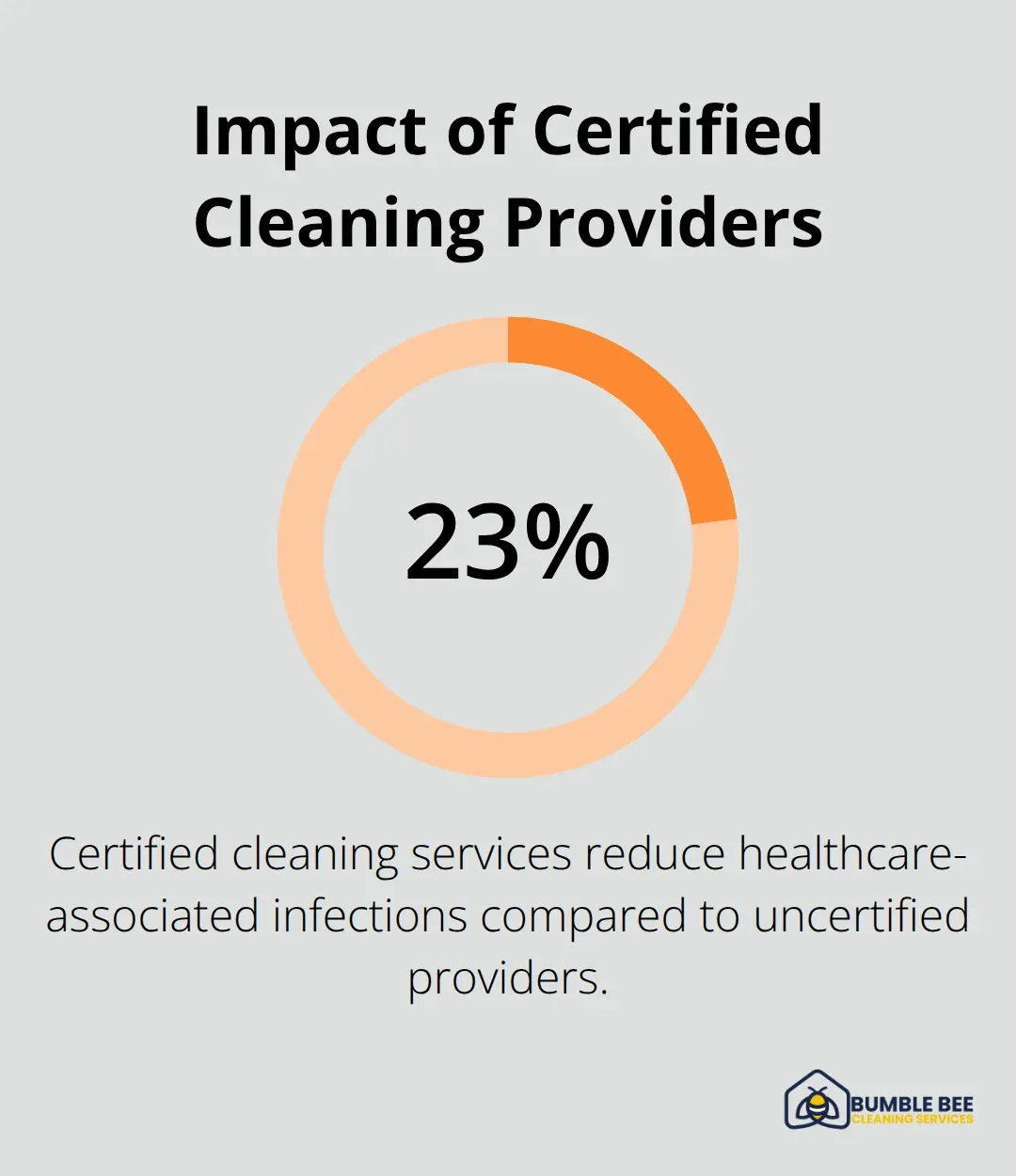
Medical cleaning service selection requires verification of specific healthcare certifications that go beyond standard commercial cleaning credentials. ARCSI certification demonstrates advanced training in infection control procedures, while IICRC credentials validate technical expertise in specialized cleaning methods. The Association for Professionals in Infection Control and Epidemiology reports that facilities with certified cleaning services reduce healthcare-associated infections by 23% compared to those with uncertified providers.
Staff training documentation must include bloodborne pathogen protocols, chemical safety procedures, and pathogen-specific cleaning techniques. Competency assessments occur every six months with documented results. Service providers must demonstrate their staff complete ongoing education programs that address evolving healthcare regulations and infection control best practices.
Experience with Healthcare Regulations
Healthcare cleaning partners need demonstrated experience with Joint Commission surveys, CMS inspections, and state health department audits. Request client references from similar facilities and review their infection control violation history through state licensing boards. Companies with healthcare experience maintain current knowledge of regulations like FDA Food Code updates and OSHA’s revised Bloodborne Pathogens Standard interpretations.
Partners should provide detailed compliance documentation that includes staff training records, chemical safety data sheets, and protocol update procedures. This documentation demonstrates their commitment to regulatory adherence and protects your facility from potential violations during inspections.
Quality Assurance and Documentation Processes
Effective medical cleaning services implement ATP monitoring systems that measure organic contamination in real-time with pass/fail thresholds below 250 RLU for patient care areas. Documentation systems track cleaning completion times, product usage rates, and surface contamination levels with electronic reporting capabilities (providing immediate access to compliance data).
Monthly quality audits include microbial sampling of high-risk surfaces with results compared against established benchmarks. Service providers must maintain detailed incident reports for exposure events, product failures, and protocol deviations. These reports include corrective action plans that prevent recurrence and protect patient safety through systematic quality improvements.
Final Thoughts
Medical facilities face specialized requirements that directly impact patient safety and regulatory compliance. Healthcare-associated infections affect 1 in 31 hospital patients, which makes proper environmental protocols a patient safety imperative rather than just maintenance work. Success depends on comprehensive staff training in bloodborne pathogen procedures, documented compliance with CDC guidelines and Joint Commission standards, and consistent quality monitoring through ATP testing and microbial sampling.
Healthcare facility managers must evaluate their current protocols against OSHA requirements and CDC recommendations. Facilities need detailed schedules, verified chemical contact times, and documented procedures for regulatory inspections. Partner with certified services that demonstrate healthcare experience through ARCSI credentials and documented infection control training (which reduces healthcare-associated infections by 23% compared to uncertified providers).
We at Bumble Bee Cleaning Services provide professional medical facility cleaning with comprehensive documentation systems that support your compliance requirements. Regular audits of effectiveness, staff competency assessments, and protocol updates based on current research form the foundation of effective infection prevention programs. These programs protect both patients and healthcare workers through systematic quality improvements and regulatory adherence.
