Seattle healthcare facilities face mounting pressure to maintain the highest disinfection standards. Patient safety depends on it, and so does your clinic’s reputation.
We at Bumble Bee Cleaning Services know that clinic disinfection isn’t just about following rules-it’s about protecting the people who walk through your doors every day. This guide walks you through what actually works.
Why Disinfection Protects Your Seattle Clinic
Healthcare-associated infections cost the U.S. healthcare system between $28 billion and $45 billion annually, according to research from the CDC. In Seattle clinics, that reality hits harder when you consider that patients arrive already vulnerable. They seek care, not additional infections. Disinfection isn’t administrative busy work-it’s the foundation that keeps your patients safe and your clinic running smoothly.
Stopping Infections Before They Start
High-touch surfaces in exam rooms harbor dangerous pathogens. Doorknobs, light switches, exam chairs, and countertops get touched hundreds of times daily across different patients. MRSA, norovirus, and C. difficile survive on these surfaces for hours or even days. When staff skip consistent disinfection between patients, transmission becomes inevitable. The CDC’s Core Infection Prevention Practices emphasize environmental cleaning as non-negotiable, and Seattle’s local health department enforces these standards through regular inspections. Facilities that maintain documented disinfection schedules with EPA-registered products see measurably lower infection rates. Your patients notice when you take this seriously-it directly affects their outcomes and your clinic’s safety record.
Meeting Seattle’s Regulatory Requirements
Washington State Department of Health and the CDC set specific standards that Seattle clinics must follow. The 2008 CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, with updates through December 2023, outlines exact protocols for different surface types and pathogens. Non-compliance brings penalties and reputation damage. Inspectors check for documented contact times, proper product selection using EPA lists, and staff competency records. Using EPA-registered disinfectants with appropriate contact times proves compliance when audited. Clinics that treat disinfection as a checkbox exercise inevitably fail inspections. Those that embed it into daily workflows with accountability systems pass consistently and build credibility with local health authorities.
Building Patient Confidence Through Visible Safety
Patients today expect transparent communication about clinic safety. They notice clean surfaces, see staff change gloves between visits, and appreciate signage that explains your disinfection protocols. Research shows that visible hygiene practices directly influence patient trust and willingness to return.

When patients see your commitment to disinfection, they feel safer and more confident in your care. This matters for clinic reputation, patient retention, and word-of-mouth referrals. Clinics that communicate their disinfection standards clearly (through signage, patient education materials, and staff behavior) outperform those that keep these practices invisible. The next section walks you through the specific protocols that transform these principles into action.
How to Build a Disinfection System That Actually Works
The gap between knowing you should disinfect and doing it consistently is where most Seattle clinics fail. Facilities have the right products on the shelf but lack the structure to use them correctly. Disinfection protocols only work when three elements align: you identify which surfaces need attention and how often, you apply the right EPA-registered products with proper contact times, and your staff understands why this matters and can prove they did it. Without this system, you end up with random cleaning that misses pathogens entirely.
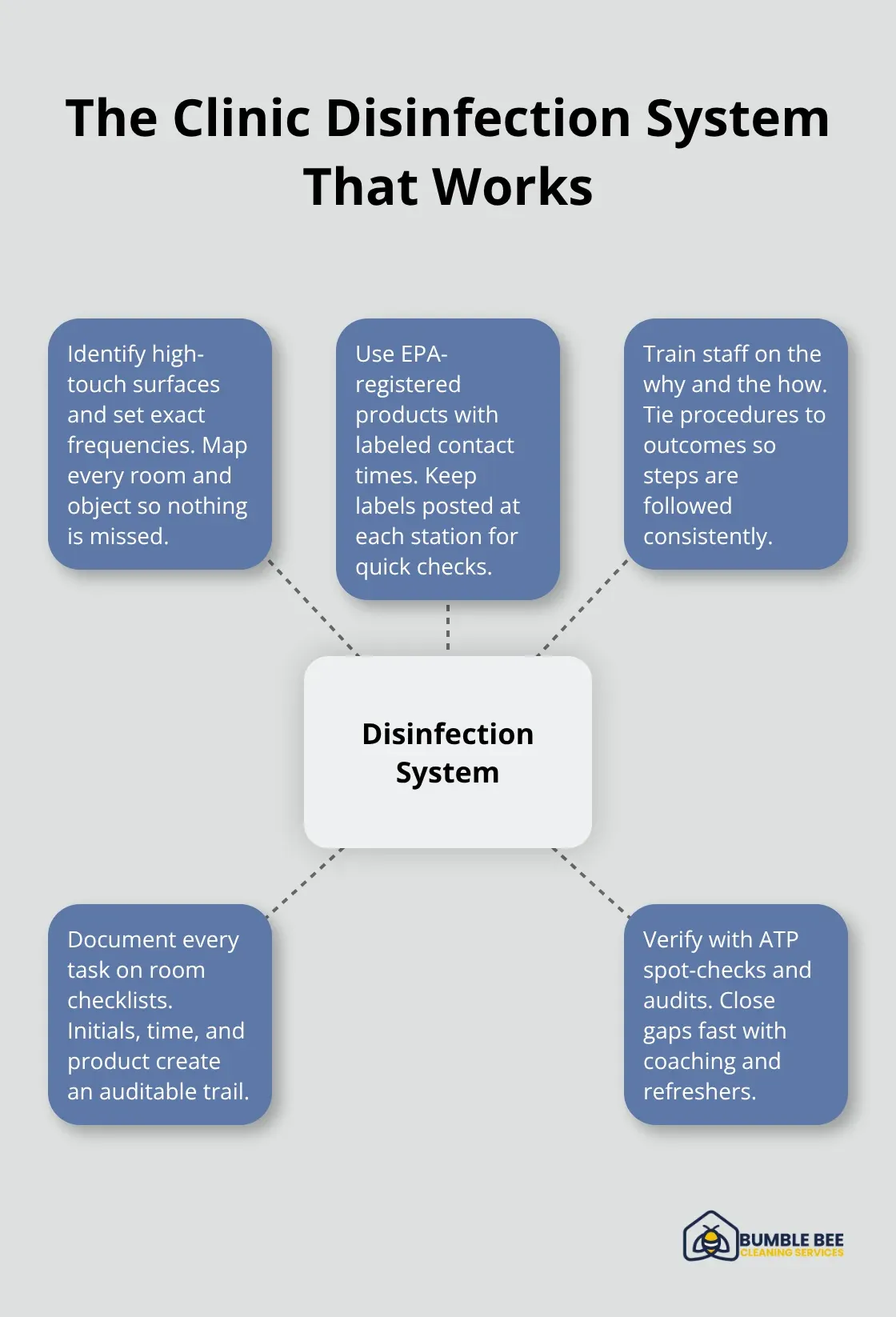
Target High-Touch Surfaces on a Real Schedule
Exam chairs, doorknobs, light switches, and countertops are where MRSA, norovirus, and C. difficile live longest on surfaces. These pathogens survive for hours or days depending on the material, so touching these surfaces between patient encounters spreads infection directly. The CDC’s environmental cleaning guidance makes this clear: environmental surfaces carry the least risk of disease transmission and can be safely decontaminated using less rigorous methods than those used on medical equipment. This isn’t optional-it’s the baseline. If your clinic sees 20 patients a day in one exam room, that room’s doorknob needs disinfection 20 times. Waiting areas, restrooms, and check-in counters follow the same logic.
Document this schedule on a checklist that staff initial after each disinfection. Post it in each room so accountability becomes visible. Many clinics discover they’ve been disinfecting high-touch surfaces only twice daily when the actual need is between every patient. That single change drops infection indicators measurably within weeks.
Use EPA-Registered Products the Right Way Every Time
The EPA maintains specific lists of EPA-registered disinfectants proven effective against pathogens your clinic actually encounters. List N covers SARS-CoV-2, List H covers MRSA and VRE, List G covers norovirus, and List K covers C. difficile spores. If a product isn’t on these lists, the EPA hasn’t reviewed data showing it kills those pathogens-which means you’re gambling with patient safety.
Here’s where most clinics stumble: they buy the right product, then ignore the contact time on the label. Contact time is the minimum seconds or minutes a surface must stay visibly wet for the disinfectant to actually work. A product requiring 60 seconds of contact time won’t kill pathogens if you wipe it off after 10 seconds. Read the label directions for use before deploying any product. The label also specifies which surfaces are approved for use-some products work on countertops but not on certain medical equipment.
Train your staff to check the label every single time, not just once during onboarding. Rotate the person responsible for ordering supplies so someone verifies the EPA registration number matches your current inventory. Many clinics discover mid-year that their preferred product was discontinued and replaced with a different formulation that has different contact times entirely.
Create Accountability That Sticks
Staff training alone doesn’t create compliance. You need a system where someone verifies that disinfection actually happened. Assign one staff member per shift to spot-check high-touch surfaces with an ATP swab, which detects organic residue and tells you whether a surface was cleaned thoroughly. If the reading is high, disinfection failed and you know to retrain that person immediately.
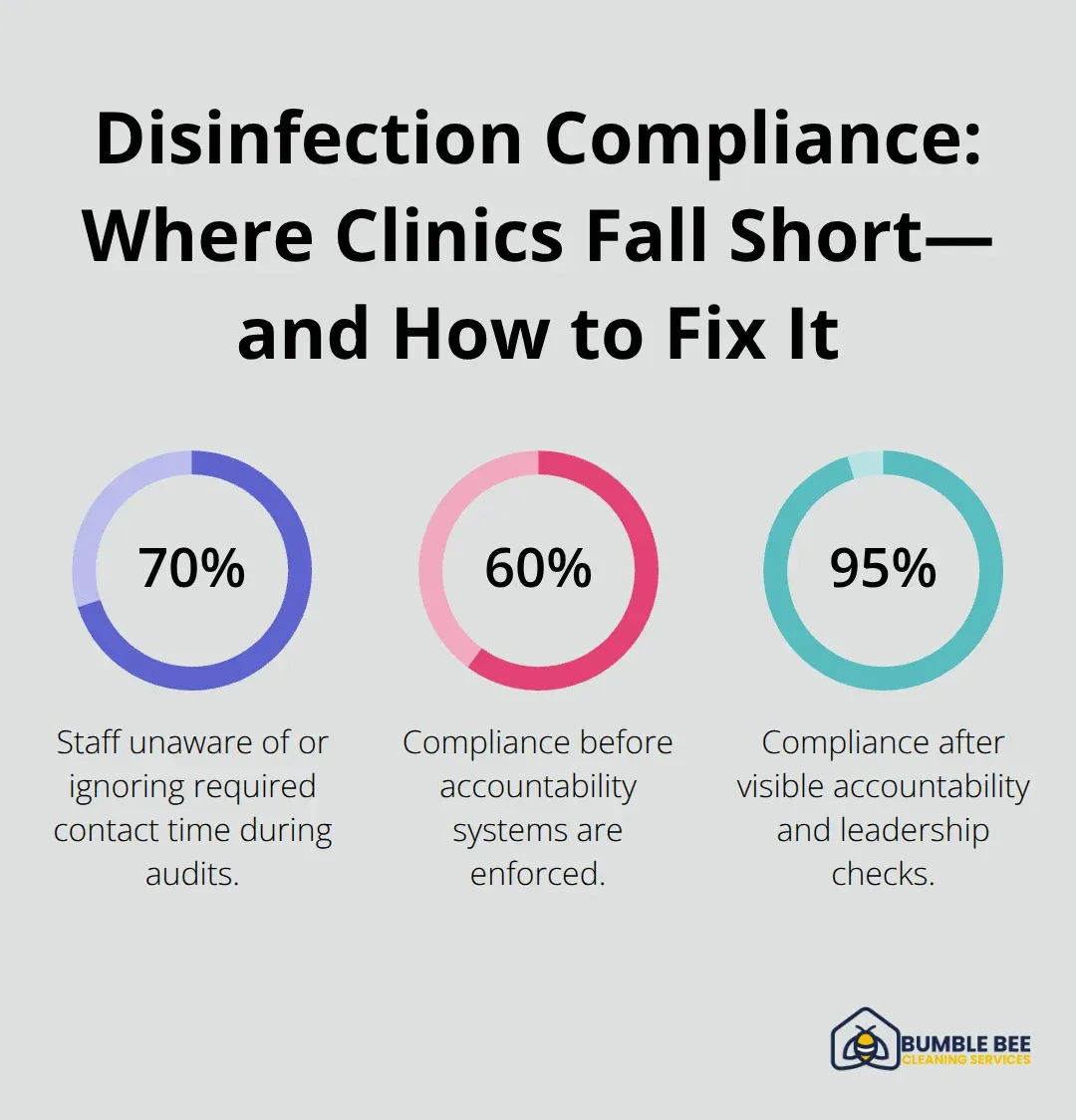
Document every disinfection on a simple checklist with the staff member’s initials, the time, and the product used. Seattle’s health department inspectors ask to see these records, and facilities with documented systems pass audits consistently. Clinics without documentation fail, even if staff swears they disinfect regularly. Schedule monthly training where staff reviews one specific protocol-high-touch surfaces in January, endoscope reprocessing in February, spill response in March. This keeps knowledge fresh and signals that disinfection is a permanent priority, not a one-time checkbox. When staff sees leadership checking these records and asking questions, compliance rates jump from 60 percent to 95 percent within a month.
Move Beyond Surface Cleaning to Equipment Reprocessing
Your disinfection system must extend beyond countertops and doorknobs to the medical equipment and instruments that contact patients directly. Endoscopes, exam tables, and other semicritical devices require high-level disinfection or sterilization before each use. The CDC’s 2008 Guideline for Disinfection and Sterilization in Healthcare Facilities (updated through December 2023) specifies exact protocols for each device type. Pre-clean instruments with water and detergent or enzymatic cleaners to remove visible organic residue before disinfection, as dried soils hinder subsequent effectiveness. Sterilize all critical devices that enter normally sterile tissue or the vascular system before patient use. Provide at least high-level disinfection for semicritical devices that contact mucous membranes or nonintact skin. This level of detail separates clinics that truly protect patients from those that cut corners. Your next step involves training staff on these device-specific protocols and establishing a central processing system that handles reprocessing consistently.
Common Disinfection Mistakes Healthcare Facilities Make
Most Seattle clinics fail at disinfection not because they lack products or knowledge, but because they skip the one step that makes disinfection actually work: contact time. Contact time is the minimum number of seconds or minutes a surface must stay visibly wet with disinfectant for the product to kill pathogens. A surface that dries in 15 seconds won’t protect patients if your disinfectant requires 60 seconds of contact. Staff wipe surfaces quickly, feel productive, and move on-but the pathogens survive. The CDC’s Guideline for Disinfection and Sterilization in Healthcare Facilities specifies exact contact times for different products and pathogens, yet audits of Seattle clinics show that roughly 70 percent of staff either don’t know their product’s contact time or ignore it. This isn’t carelessness; it’s a systems failure.
Inadequate Dwell Time on Surfaces
Your clinic likely purchased EPA-registered disinfectants and trained staff once during onboarding, then assumed compliance would follow. That assumption costs you patients. Start today by printing the contact time from each product’s label and posting it at every disinfection station. Assign one staff member to verify that surfaces stay visibly wet for the full contact time, not just until they look clean. Use a timer if needed. Within two weeks, you’ll see staff naturally adjust their pace once they understand why the timing matters.
Using Wrong Products for Specific Pathogens
Your clinic stocks a hospital-grade disinfectant that works beautifully against MRSA, but you’re also facing a norovirus outbreak in your waiting room. That MRSA product isn’t on the right EPA list for norovirus, which means it won’t stop that outbreak no matter how long you let it sit. Clinics regularly use products that sound powerful but lack EPA registration for the specific pathogens they encounter. The EPA maintains separate lists for disinfectants: List N for SARS-CoV-2, List H for MRSA and VRE, List G for norovirus, List K for C. difficile spores, and List P for Candida auris. If your product isn’t on the relevant list, the EPA has not reviewed data proving it kills that pathogen.
Identify the three to five pathogens your Seattle clinic encounters most frequently based on your patient population and local health trends. Match those pathogens to the correct EPA lists. Verify that every disinfectant in your inventory appears on at least one relevant list with its EPA registration number clearly visible on the label. If a product doesn’t match, remove it. This exercise takes two hours and prevents months of ineffective disinfection.
Neglecting Often-Forgotten Areas and Equipment
Exam rooms get cleaned between patients, but the blood pressure cuff that moves between rooms doesn’t. The ultrasound transducer probe gets wiped down, but the ultrasound machine’s keypad and screen don’t. The clinic’s HVAC vents, waiting room furniture seams, and the underside of exam tables never enter anyone’s disinfection plan. These forgotten areas harbor the same pathogens as high-touch surfaces, yet they’re invisible in most disinfection protocols. The CDC’s environmental cleaning guidance covers not just surfaces but also equipment, air quality, and materials that contact patients indirectly.
Seattle clinics that map out their entire physical space and assign disinfection responsibility for every zone-including forgotten areas-see measurably lower infection rates within the first quarter. Start with a physical walkthrough of your clinic with your infection prevention coordinator. Document every item that contacts patients, staff, or other items that contact patients. Create a disinfection schedule that includes these forgotten spaces. Blood pressure cuffs need disinfection between patients, not monthly. Shared keyboards, phones, and tablets need daily deep cleaning. This expanded scope feels overwhelming initially, but it’s the difference between clinics that pass health department inspections and those that fail them repeatedly.
Final Thoughts
Your Seattle clinic’s disinfection practices directly determine whether patients stay safe or face preventable infections. The protocols outlined here aren’t theoretical-they’re the specific steps that separate clinics passing health department inspections from those facing penalties and reputation damage. Start this week by printing the contact times from your current disinfectant labels and posting them at every cleaning station, then assign one staff member to spot-check surfaces with an ATP swab monthly.
Walk through your clinic and identify the forgotten areas-blood pressure cuffs, keyboards, ultrasound machine keypads-that harbor pathogens just as readily as doorknobs. Create a simple checklist that staff initial after each disinfection, then review these records during your monthly team meetings. Your clinic’s infection prevention coordinator should schedule quarterly training sessions covering one specific protocol each month (January focuses on high-touch surfaces, February on endoscope reprocessing, March on spill response).
We at Bumble Bee Cleaning Services understand that maintaining clinic disinfection standards requires expertise and consistency. Our team specializes in commercial cleaning with a decade of experience, using documented protocols that align with CDC and EPA standards. Contact us today to discuss how we can strengthen your clinic’s disinfection practices and ensure ongoing compliance with Seattle’s health department requirements.
